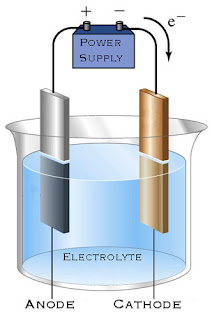
Electrolysis of Molten Compounds
- Electrolysis (with battery / electricity current) is a process of decomposition / breaking down / separation of a compound (electrolyte) into its constituent elements when electric current passes through it.
| Anode | Electrode connected to the positive terminal (+) of a battery |
| Cathode | Electrode connected to the negative terminal (-) of a battery |
| Anion | Negatively-charged ion. Example: Cl-, SO42- and O2- |
| Cation | Positively-charged ion. Example: Na+, Zn2+ and Al3+ |
| Inert electrodes | Electrodes that do not take part in chemical reactions during electrolysis | Carbon or platinum |
| Active electrodes | Electrodes that take part in chemical reactions during electrolysis | Copper or zinc |
Electrolysis of Aqueous Compounds (dissolved in water, H2O)
There are three important factors to determine the types of ions to be discharged at the electrodes.
- Positions of ions in the electrochemical series
- Concentration of ions in the solution
- Types of electrodes used
The lower the position of the ion in the electrochemical series, the easier the ion is selectively discharged.
Electrochemical series:
| Cation | Anion |
| K+ | F- |
| Na+ | SO42- |
| Ca2+ | NO3- |
| Mg2+ | Cl- |
| Al3+ | Br- |
| Zn2+ | I- |
| Fe2+ | OH- |
| Sn2+ | |
| Pb2+ | |
| H+ | |
| Cu2+ | |
| Hg+ | |
| Ag+ | |
| Au+ |
2. Effect of concentration of ions in the solution
The concentration of a particular type of ion is high = ion more likely to be discharged in electrolysis.
3. Types of electrodes used in the electrolysis
There are 2 important notes:
- Inert electrodes: Carbon and platinum (Both of these electrodes do not react with the electrolytes or products of electrolysis)
- Active electrodes: Silver, copper and nickel (Active anode ionises and concentration of cations in the electrolyte does not change)